Electrons in the Lewis Dot Structure? ZDraw Lewis Structures for O 2 and N 2. 14 Covalent Bond zThere is an optimum distance between atoms in a covalent bond. This is the bond length: calculated by adding the radii of two atoms. Strengths of Covalent Bonds zCloser nuclei result in a stronger bond. Shorter bond = stronger bond. . Lewis structures incorporate an atom’s formal charge, which is the charge on an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms. Lewis dot diagrams are often employed to visualize the covalent bonding between atoms in a compound.
- Lewis Dot Structure Covalent Bonds Calculator Answer
- Covalent Lewis Dot Structure
- Covalent Bonds Lewis Dot Diagram
Could someone explain the lewis structure diagram of covalent compound Al2Cl6?

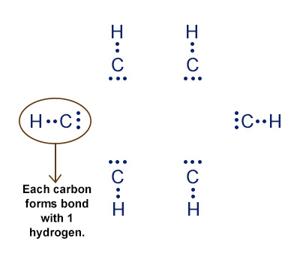
Also, why is Al2Cl6 (aluminium chloride) covalent?
Here's what we have to know for school but I don't know how it works. What do the arrows mean?
Also, why is Al2Cl6 (aluminium chloride) covalent?
Here's what we have to know for school but I don't know how it works. What do the arrows mean?
Using Lewis Dot Symbols to Describe Covalent Bonding The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence. Covalent bonds are between. Preview this quiz on Quizizz. Lewis Dot Structures, Covalent and Ionic DRAFT. 10th - 11th grade. Electrons in the Lewis Dot Structure? ZDraw Lewis Structures for O 2 and N 2. 14 Covalent Bond zThere is an optimum distance between atoms in a covalent bond. This is the bond length: calculated by adding the radii of two atoms. Strengths of Covalent Bonds zCloser nuclei result in a stronger bond. Shorter bond = stronger bond.

Lewis Dot Structure Covalent Bonds Calculator Answer
1 Answer
Explanation:
Chlorine has 17 electrons, but 10 of those are in the orbitals of the lower energy levels. (
These are completely enveloped by the larger
The other seven are distributed in the
for more info about Hybridisation, here's a good link:
In our case the
(Picture courtesy of https://en.wikipedia.org/wiki/Orbital_hybridisation)
Each orbital can contain, and indeed strives to contain, 2 electrons (
Chlorine thus has 7 electrons in the 4
The fourth one, the one that contains the single, unpaired
Aluminium has only 3 electrons: 2 in the
Chlorine has a rather high Electronegativity, which means that it pulls rather hard at electrons from other atoms (from each other, and from other elements.
It is a tug-of-war that the Aluminium atom is threatening to lose, leaving it rather
At the same time, the Chlorine atoms are satisfied in their hunger for electrons, in fact they don't want any more because they have a full set of 8!
Because of this 'diminished appetite' on the side of the Chlorine atoms, and the increased hunger on the part of the Aluminium atom, The bond is formed by BOTH of the Chlorine electrons.
This is the explanation for the arrow circled by Aqua:
In
Hope this helps...
PS: By the way, it is covalent because the bonds are created by sharing of electrons between the two atoms.
Related questions
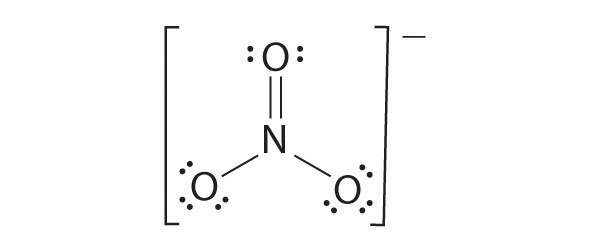
Formal charge:
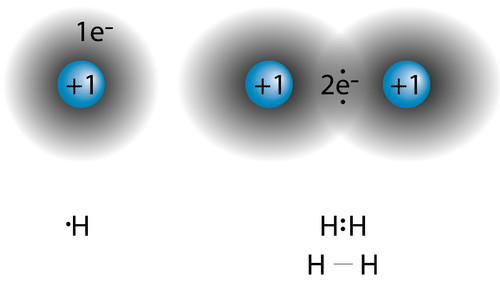
Let us draw the Lewis structure for carbon dioxide.
1. Skeletal structure
2. Total number of valence electrons in CO2
=[1 x 4(carbon)] +[2 x 6(oxygen)] = 4+ 12 = 16
3. Draw single bonds between atoms. Two bonds can be drawn as shown in the figure for CO2 which accounts for four electrons (2 bond pairs).
4. Distribute the remaining twelve electrons (16 - 4= 12) as six lone pairs starting from most electronegative atom, the oxygen. Six lone pairs are distributed to the two terminal oxygens (three each) to satisfy their octet.
5. Verify weather all the atoms have octet configuration. In the above distribution, the central carbon has two pair short for octet. Therefore, to satisfy the octet rule two lone pairs from one oxygen or one pair from each oxygen can be moved to form multiple bonds, leading the formation of two possible structures for carbon dioxide as shown below
Similarly, the Lewis structure for many molecules drawn using the above steps gives more than one acceptable structure. Let us consider the above mentioned two structures of carbon dioxide.
Which one the above forms represents the best distribution of electrons in the molecule. To find an answer, we need to know the formal charge of each atom in the Lewis structures. Formal charge of an atom in a molecule, is the electrical charge difference between the valence electron in an isolated atom and the number of electrons assigned to that atom in the Lewis structure.
Where,
Nv- Number of valence electron of atom in its isolated state.
Nl - Number of electrons present as lone pairs around the atom in the Lewis structure
Nb - Number of electrons present in bonds around the atom (bond pairs) in the Lewis structure]
Now let us calculate the formal charge on all atoms in both structures,
For Structure 1,
For structure 2
Formal charge on carbon
Covalent Lewis Dot Structure
After calculating the formal charges, the best representation of Lewis structure can be selected by using following guidelines.
1. A structure in which all formal charges are zero preferred over the one with charges.
2. A structure with small formal charges is preferred over the one with higher formal charges.
3. A structure in which negative formal charges are placed on the most electronegative atom is preferred.
Covalent Bonds Lewis Dot Diagram
In case of CO2 structures, the structure one is preferred over the structure 2 as it has zero formal charges for all atoms.